BACKGROUND
Plasma cell leukemia (PCL) is a rare and aggressive plasma cell dyscrasia, associated with short survival. This disease is usually divided into primary PCL (pPCL), when occurring de novo, or secondary PCL (sPCL), when a patient previously diagnosed with multiple myeloma (MM) undergoes leukemic transformation. Because of the very low incidence of PCL, only a few cohorts have been reported and thus, information on this disease is scarce. The goal of this study was to better understand PCL, particularly in regard to the characteristics of MM before leukemic transformation and the outcomes following modern treatments in both pPCL and sPCL.
METHODS
We performed a retrospective, multicenter study of patients diagnosed with PCL between January 2005 and December 2020 in eight institutions in the Province of Québec, Canada. Patients were identified through digital medical records. The 2013 International Myeloma Working Group (IMWG) diagnostic criteria for PCL (circulating plasma cells ≥ 20% and/or ≥ 2 x 10 9/L) were used to determine eligibility. Patients were excluded if they received treatment for another malignancy after the diagnosis of PCL.
RESULTS
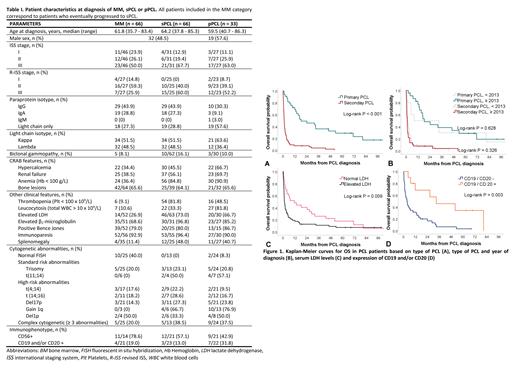
We identified 99 eligible PCL patients, of whom 33 were pPCL and 66 were sPCL. Among significant clinical characteristics, both pPCL and sPCL patients showed a high frequency of thrombocytopenia, splenomegaly, and light chain isotype (Table I). At MM diagnosis, patients who eventually progressed to sPCL were much younger (median 61.8 years) than a typical MM cohort and many of them already demonstrated markers of poor prognosis, including elevated lactate dehydrogenase (LDH), elevated ß 2-microglobulin and complex cytogenetics (Table I). The median time between initial MM diagnosis and leukemic progression was 27.3 months (IQR, 12.7 - 41.6). The median number of lines of treatment prior to transformation was 2 (range 1 - 7). Overall, autologous stem cell transplant (ASCT; n = 28) or tandem ASCT-allogeneic stem cell transplant (ASCT-alloSCT; n = 4) did not result in longer time to PCL progression when compared to those who received chemotherapy alone (31.6 vs 22.9 months, p = 0.164).
Median overall survival (OS) for pPCL and sPCL were respectively 18.3 months (95% CI, 0.0 - 39.0) and 1.2 months (95% CI, 0.9 - 1.5) (p < 0.001). When considering survival from MM diagnosis to death, the median OS for sPCL was 30.2 months (95% CI, 24.1 - 36.2). Patients with pPCL who underwent ASCT or tandem ASCT-alloSCT had a significantly longer OS than patients treated with chemotherapy alone (HR 0.27, 95% CI 0.11 - 0.64, p = 0.003). The impact of stem cell transplant on sPCL survival could not be assessed, as only one of 66 sPCL patients received this type of treatment. The median number of lines of treatment was 2 (range 1 - 9) for pPCL and 1 (range 0 - 5) for sPCL. Interestingly, patients receiving more recent regimens (2013-2020) did not have a better OS than patients receiving earlier treatments (2005-2012) (p = 0.391). When analysed separately, OS for pPCL or sPCL did not improve significantly in the later period (Figure 1). Clinical characteristics independently associated with poor outcomes were sPCL subtype and elevated LDH (respectively, HR 4.9, 95% CI 2.2 - 11.9, p < 0.001 and HR 2.4, 95% CI 1.0 - 5.7, p = 0.049). Although expression of CD19 and/or CD20 was not statistically significant in multivariable analysis, a trend towards improved survival was observed in patients expressing these markers (HR 0.4, 95% CI 0.1 - 1.1, p = 0.062).
CONCLUSION
This retrospective, multicenter study is one of the largest PCL cohorts reported and we are, to our knowledge, the first to investigate characteristics of MM patients before their transformation into sPCL. Although this subgroup is often described as a late-stage complication of heavily pretreated MM, our data suggest that sPCL originates from an already high-risk MM population rather than being the result of long-term selection of an aggressive clone. More information is however needed for early identification of MM patients at risk of PCL transformation. Finally, despite the use of newer chemotherapy regimens, we observed no improvement in OS in recent years, highlighting the urgent need for better treatment options for both pPCL and sPCL.
Disclosures
Leblanc:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; FORUS Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria. Roy:ExCellThera: Patents & Royalties, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Trudel:Janssen: Honoraria; Sanofi: Honoraria. Cote:Janssen: Honoraria, Research Funding; Sanofi: Research Funding. Lalancette:Sanofi: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Boudreault:Pfizer: Consultancy; Janssen: Consultancy; BMS: Consultancy; Sanofi: Consultancy. Lemieux-Blanchard:FORUS Therapeutics: Membership on an entity's Board of Directors or advisory committees; Apotex: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Conference ; Sanofi: Other: Conference; Pfizer: Other: Conference. Kaedbey:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; FORUS Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pavic:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; FORUS Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal